Synensys, LLC, a service-disabled veteran-owned small business (SDVOSB) safety management company, was awarded a research contract by The U.S. Food and Drug Administration (FDA) to conduct the study Improving the Reliability, Interoperability, Agility, and Quality of Laboratory Data Exchanges using System Safety Engineering Methods under an FDA Broad Agency Announcement (BAA).
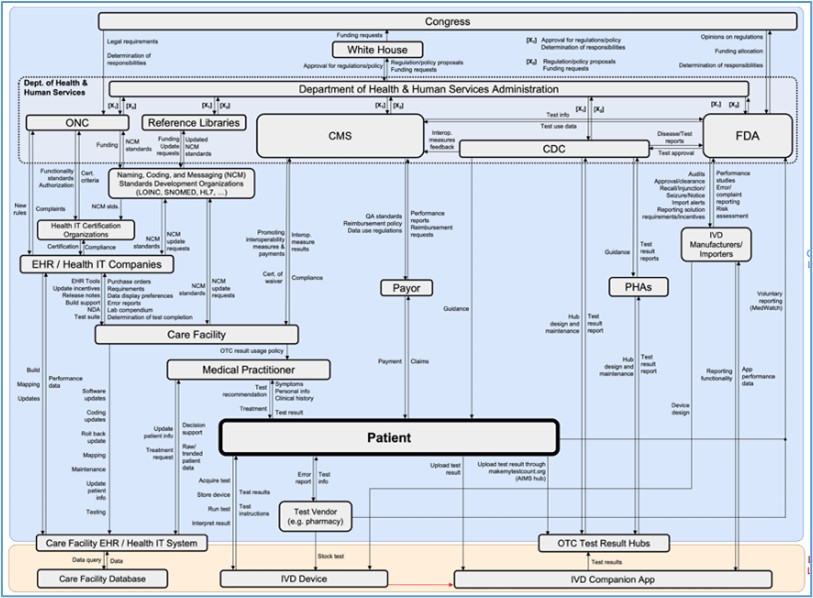
Our team, together with Massachusetts Institute of Technology (MIT), recently completed a proactive safety analysis focused on over-the-counter (OTC) and point-of-care (POC) lab data quality. Our analysis expanded a previous hazard analysis that focused on the traditional laboratory data ecosystem. The quality of the data produced from these non-traditional test settings can directly impact the quality of treatment decisions: if OTC/POC testing data is to be used more broadly for making treatment and regulatory decisions, it is imperative to identify and mitigate the hazards that can contribute to patient harm.  The same safety analysis approach to modeling and analyzing complex, adaptive systems was used to identify scenarios in the OTC/POC laboratory system design that can lead to adverse events. The method used is based on systems theory and uses an analysis technique called System-Theoretic Process Analysis (STPA). Causal factors that lead to adverse events were identified; some were present in the traditional U.S. laboratory data ecosystem, but others were unique to the OTC/POC system design. The causal factors drove the development of recommendations to mitigate the systemic factors and reduce losses throughout the system.
The same safety analysis approach to modeling and analyzing complex, adaptive systems was used to identify scenarios in the OTC/POC laboratory system design that can lead to adverse events. The method used is based on systems theory and uses an analysis technique called System-Theoretic Process Analysis (STPA). Causal factors that lead to adverse events were identified; some were present in the traditional U.S. laboratory data ecosystem, but others were unique to the OTC/POC system design. The causal factors drove the development of recommendations to mitigate the systemic factors and reduce losses throughout the system.
”"Data quality from OTC tests, especially those used during the COVID-19 pandemic, was difficult to assess due to many previously unknown system hazards," said Synensys CEO Dr. Stephen Powell, "This study provides a comprehensive analysis of the current OTC data quality and safety challenges with 8 systemic recommendations for system redesign and continuous improvement."